 Take a look at the lamp post. Bad hair day huh? It is found in Section 19 P.J. . I wonder how the authourities allow such a mess to perch over the public. It is time to clear that up and show our neatness in all that we do. That is a little too dangerous. Imagine a bird that landed up there and touching a live wire. I bet its feathers will look like that.
Take a look at the lamp post. Bad hair day huh? It is found in Section 19 P.J. . I wonder how the authourities allow such a mess to perch over the public. It is time to clear that up and show our neatness in all that we do. That is a little too dangerous. Imagine a bird that landed up there and touching a live wire. I bet its feathers will look like that.How's The Air?
 It's clear skies today at 5.30 pm! A very drastic difference from the condition this morning. At about 9.30 am, the Telekom Tower was hardly visible from Section 12. The air was choking and we prayed that the school would do us some good by aborting all classes immediately. Ah, and once again, the lime juice has worked its miracle again! My throat feels fine after the drink even though the acidity of the fruit was a little too high. Hint: don't ask for kurang gula!
It's clear skies today at 5.30 pm! A very drastic difference from the condition this morning. At about 9.30 am, the Telekom Tower was hardly visible from Section 12. The air was choking and we prayed that the school would do us some good by aborting all classes immediately. Ah, and once again, the lime juice has worked its miracle again! My throat feels fine after the drink even though the acidity of the fruit was a little too high. Hint: don't ask for kurang gula!The Overexcited Chemistry Procedure
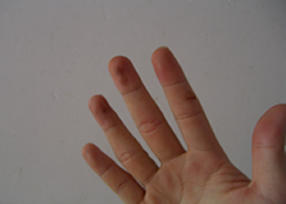 The chemical test today was a success! We managed to pin point the existence of the chloride ion in the unidentified salt. First, we saw the misty fumes of HCl when we added sulphuric acid. Then the silver nitrate test gave us the white precipitate that dissolves in aqueous ammonia. Finally, we did a brave test (The T.L.K. style). We added the solid salt with potassium dichromate (VII) with concentrated sulphuric acid. Berjaya! Red fumes which came out confirmed the chloride ion. However, it kept generating. Frantic actions brought the T.L.K. waving the test tube around, with the thing still smoking. Then, the smoke died as the chemicals were drowned in water. Ah, the experience! Now, we have to monitor ourselves as we could have breathed in much toxins. And look at my hands. Either the sulphuric acid nibbled at my fingers or the silver nitrate oxidised on it. Scary...
The chemical test today was a success! We managed to pin point the existence of the chloride ion in the unidentified salt. First, we saw the misty fumes of HCl when we added sulphuric acid. Then the silver nitrate test gave us the white precipitate that dissolves in aqueous ammonia. Finally, we did a brave test (The T.L.K. style). We added the solid salt with potassium dichromate (VII) with concentrated sulphuric acid. Berjaya! Red fumes which came out confirmed the chloride ion. However, it kept generating. Frantic actions brought the T.L.K. waving the test tube around, with the thing still smoking. Then, the smoke died as the chemicals were drowned in water. Ah, the experience! Now, we have to monitor ourselves as we could have breathed in much toxins. And look at my hands. Either the sulphuric acid nibbled at my fingers or the silver nitrate oxidised on it. Scary...
3 comments:
Brown stains...AgNO3!!!!!
I got that during my last Chemistry practical...
Wow to confirm the Cl- ion you guys have to do so many things? I thought it was only acidify soln with HNO3, then add AgNO3, then excess NH3...
Actually, we were kinda adventurous so we want to confirm the test =)
Dude.. its not adventurous ok? I decided to do it cos' we all want to confirm it further. And it was you who said :"Add some more suphuric acid"
Post a Comment